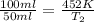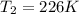Chemistry, 29.01.2020 23:57 Sumitco9578
If a volume of nitrogen gas at 452 k decreases from 100.0 ml to 50.0 ml, what is the final kelvin temperature? assume pressure remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
If a volume of nitrogen gas at 452 k decreases from 100.0 ml to 50.0 ml, what is the final kelvin te...
Questions

Health, 22.07.2019 01:00

English, 22.07.2019 01:00


History, 22.07.2019 01:00

Geography, 22.07.2019 01:00







Mathematics, 22.07.2019 01:00






Biology, 22.07.2019 01:00


History, 22.07.2019 01:00

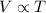
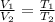
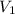 = initial volume of gas = 100 ml
= initial volume of gas = 100 ml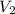 = final volume of gas = 50 ml
= final volume of gas = 50 ml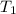 = initial temperature of gas = 452 K
= initial temperature of gas = 452 K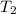 = final temperature of gas = ?
= final temperature of gas = ?