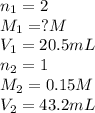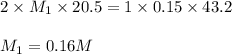Atitration was performed in a lab situation. h2so4 was titrated with naoh. the following data was collected: ml of naoh used = 43.2 ml concentration naoh = 0.15 m ml h2so4 = 20.5 ml notice that h2so4 releases 2 h+ per mole. what is the concentration of h2so4? 0.036 m 0.16 m 0.63 m 6.3 m

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Atitration was performed in a lab situation. h2so4 was titrated with naoh. the following data was co...
Questions












Mathematics, 31.05.2021 15:10



Business, 31.05.2021 15:10



Mathematics, 31.05.2021 15:10


Chemistry, 31.05.2021 15:10

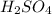 comes out to be 0.16 M.
comes out to be 0.16 M.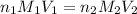
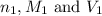 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 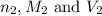 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.