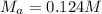Chemistry, 18.01.2020 09:31 Sumthin4695
A29.00 ml sample of an unknown h3po4 solution is titrated with a 0.130 m naoh solution. the equivalence point is reached when 27.73 ml of naoh solution is added.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1


Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
A29.00 ml sample of an unknown h3po4 solution is titrated with a 0.130 m naoh solution. the equivale...
Questions

Mathematics, 17.11.2020 01:30


Chemistry, 17.11.2020 01:30




Mathematics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Biology, 17.11.2020 01:30


Mathematics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Social Studies, 17.11.2020 01:30


Mathematics, 17.11.2020 01:30

History, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

Mathematics, 17.11.2020 01:30

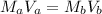 which is the titration formula. M is molarity (a is of acid and b is of base) and V is volume in mL (a is of acid and b is of base). Plugging in gives us
which is the titration formula. M is molarity (a is of acid and b is of base) and V is volume in mL (a is of acid and b is of base). Plugging in gives us 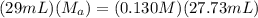 . Solving gives us
. Solving gives us