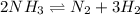Chemistry, 18.09.2019 01:00 krystalScott17
The reaction below is at dynamic equilibrium. mc004-1.jpg which statement is true for the equilibrium system? the concentration of nh3 is greater than the concentration of n2. the concentration of nh3 equals the concentration of n2. the rate of the forward reaction equals the rate of the reverse reaction. the rate of the forward reaction is greater than the rate of the reverse reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1


Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
The reaction below is at dynamic equilibrium. mc004-1.jpg which statement is true for the equilibriu...
Questions



Mathematics, 13.02.2020 00:13

Mathematics, 13.02.2020 00:13



History, 13.02.2020 00:13





Biology, 13.02.2020 00:13





Mathematics, 13.02.2020 00:13