

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
For the reaction, calculate how many moles of the product form when 0.048 mol of o2 completely react...
Questions



Mathematics, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30




Arts, 10.05.2021 17:30

Social Studies, 10.05.2021 17:30




Mathematics, 10.05.2021 17:30


Mathematics, 10.05.2021 17:30

Mathematics, 10.05.2021 17:30


Social Studies, 10.05.2021 17:40

Mathematics, 10.05.2021 17:40

Social Studies, 10.05.2021 17:40

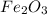 form are 0.032 moles.
form are 0.032 moles. = 0.048 mole
= 0.048 mole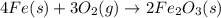
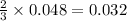 moles of
moles of 

