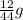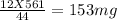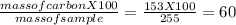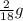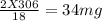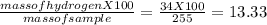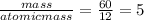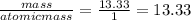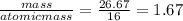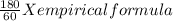Chemistry, 26.01.2020 16:31 jaymee2904p88tgh
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o, and you find that 561 mg of co2 is produced, along with 306 mm of h2o.
if the substance contains only c, h, and o, what is the empirical formula
if the molar mass of the compound is 180 g/mol what is the molecular formula of the compound

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
You perform a combustion analysis on a 255 mg sample of a substance that contains only c, h, and o,...
Questions

Mathematics, 10.09.2021 05:40


Advanced Placement (AP), 10.09.2021 05:40



Mathematics, 10.09.2021 05:40

Mathematics, 10.09.2021 05:40


English, 10.09.2021 05:40



English, 10.09.2021 05:40


Chemistry, 10.09.2021 05:40


Mathematics, 10.09.2021 05:40



Health, 10.09.2021 05:40