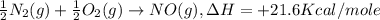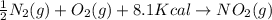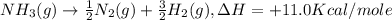Chemistry, 09.01.2020 06:31 MysteryDove12
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57.83 kcal/mole
-½n2(g) + ½o2(g) → no(g), δh = +21.6 kcal/mole
-½n2(g) + o2(g) + 8.1 kcal → no2(g)
-½n2(g) + 3/2h2(g) → nh3(g) + 11.0 kcal/mole
-nh3(g) → ½n2(g) + 3/2h2(g), δh = +11.0 kcal/mole

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Clouds form when water vapor to form small droplets. a. humidifies b. condenses c. evaporates d. precipitates
Answers: 2


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
Select all of the following reactions that are endothermic.
-h2(g) + ½o2(g) → h2o(g), δh = -57...
-h2(g) + ½o2(g) → h2o(g), δh = -57...
Questions









Mathematics, 02.07.2019 23:50



Mathematics, 02.07.2019 23:50





History, 02.07.2019 23:50

English, 02.07.2019 23:50

Mathematics, 02.07.2019 23:50

English, 02.07.2019 23:50