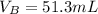Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
How many ml of 0.150m naoh (base) solution are required to neutralize 35.0ml of 0.220m hcl (acid) so...
Questions





History, 02.11.2020 22:30




Biology, 02.11.2020 22:30

Physics, 02.11.2020 22:30

Physics, 02.11.2020 22:30


English, 02.11.2020 22:30

English, 02.11.2020 22:30


Mathematics, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30

English, 02.11.2020 22:30

Physics, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30