
Chemistry, 27.12.2019 18:31 chandranewlon
What is the maximum amount of moles of p2o5 that can theoretically be made from 136 g of p4 and excess oxygen

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
What is the maximum amount of moles of p2o5 that can theoretically be made from 136 g of p4 and exce...
Questions

Mathematics, 11.01.2021 17:40





English, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40



Chemistry, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40





History, 11.01.2021 17:40


Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

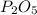 theoretically made is 2.194 moles.
theoretically made is 2.194 moles.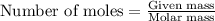
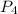 = 136 g
= 136 g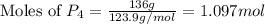
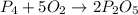
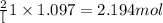 of
of 

