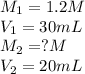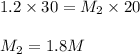Chemistry, 23.11.2019 12:31 Mariela2699
If it requires 30.0 milliliters of 1.2 molar hcl to neutralize 20.0 milliliters of naoh, what is the concentration of the naoh solution? balanced equation: naoh hcl nacl h2o 0.60 m naoh 0.80 m naoh 1.3 m naoh 1.8 m naoh

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
If it requires 30.0 milliliters of 1.2 molar hcl to neutralize 20.0 milliliters of naoh, what is the...
Questions

Biology, 21.04.2021 22:10


Spanish, 21.04.2021 22:10



Mathematics, 21.04.2021 22:10

Spanish, 21.04.2021 22:10

English, 21.04.2021 22:10

German, 21.04.2021 22:10

Chemistry, 21.04.2021 22:10

History, 21.04.2021 22:10

Mathematics, 21.04.2021 22:10


Mathematics, 21.04.2021 22:20





History, 21.04.2021 22:20


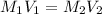
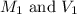 are the molarity and volume of HCl.
are the molarity and volume of HCl.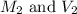 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.