Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Calculate the amount of heat required to completely sublime 28.0 g of solid dry ice (co2 at its subl...
Questions

Mathematics, 07.12.2020 17:30


Mathematics, 07.12.2020 17:30

Social Studies, 07.12.2020 17:30




Computers and Technology, 07.12.2020 17:30


Mathematics, 07.12.2020 17:30





Social Studies, 07.12.2020 17:30

English, 07.12.2020 17:30


Mathematics, 07.12.2020 17:30

English, 07.12.2020 17:30

Mathematics, 07.12.2020 17:30

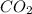 is 20.5 kJ.
is 20.5 kJ.