
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these elements compare when they form bonds?
1-both a fluorine atom and a bromine atom lose one electron, and both atoms become stable.
2-a fluorine atom becomes stable by losing one electron, but a bromine atom cannot become stable by losing only one electron.
3-both a fluorine atom and a bromine atom gain one electron, and both atoms become stable.
4-a fluorine atom becomes stable by gaining one electron, but a bromine atom cannot become stable by gaining only one electron.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Fluorine (f) and bromine (br) are in the same group on the periodic table. how do atoms of these ele...
Questions

History, 18.11.2020 19:20


Mathematics, 18.11.2020 19:20







Health, 18.11.2020 19:20

Biology, 18.11.2020 19:20





Mathematics, 18.11.2020 19:20



Physics, 18.11.2020 19:20

Biology, 18.11.2020 19:20

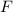 and bromine,
and bromine, 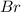 are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as
are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as 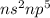 that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons.
that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons. 

