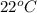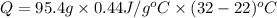Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
The specific heat of nickel is 0.44 j/g*⁰c. how much energy needed to change the temperature of 95.4...
Questions



Biology, 11.07.2021 05:40

Geography, 11.07.2021 05:40

Arts, 11.07.2021 05:40

Mathematics, 11.07.2021 05:40

Health, 11.07.2021 05:40

Mathematics, 11.07.2021 05:40

Health, 11.07.2021 05:50

Biology, 11.07.2021 05:50

Engineering, 11.07.2021 05:50

Mathematics, 11.07.2021 05:50


Biology, 11.07.2021 05:50




Mathematics, 11.07.2021 05:50

Mathematics, 11.07.2021 05:50

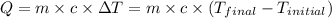
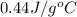
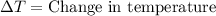
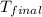 = final temperature =
= final temperature = 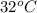
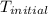 = initial temperature =
= initial temperature =