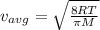Imagine you have two containers of gas, one labeled gas a and one labeled gas b. gas a has an average speed of 700 m/s and gas b has an average speed of 1100 m/s. both containers are being held at the same temperature. how could you match the average speeds of gas a and gas b?
a. reduce the volumes of both containers.
b. raise the temperature of gas b.
c. lower the temperature of gas a.
d. lower the temperature of gas b.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
Imagine you have two containers of gas, one labeled gas a and one labeled gas b. gas a has an averag...
Questions

Spanish, 22.05.2021 04:30


Mathematics, 22.05.2021 04:30


Social Studies, 22.05.2021 04:30


Mathematics, 22.05.2021 04:30



Mathematics, 22.05.2021 04:30


English, 22.05.2021 04:30



Mathematics, 22.05.2021 04:30

Mathematics, 22.05.2021 04:30

Mathematics, 22.05.2021 04:30


Mathematics, 22.05.2021 04:30