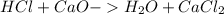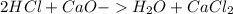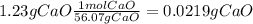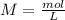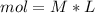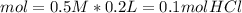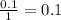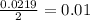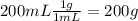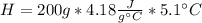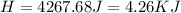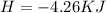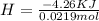During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes a temperature increase of 5.10 °c. assuming the solution\'s final volume is 200.0 ml, the density if 1.00 g/ml, and the heat capacity is 4.184 j/(g·°c, calculate the heat of the reaction, ? hrxn.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
During an experiment, a student adds 1.23 g of cao to 200.0 ml of 0.500 m hcl. the student observes...
Questions

Mathematics, 05.07.2019 02:50


Mathematics, 05.07.2019 02:50

Mathematics, 05.07.2019 02:50

Arts, 05.07.2019 02:50

Social Studies, 05.07.2019 02:50

Mathematics, 05.07.2019 02:50


Biology, 05.07.2019 02:50



Mathematics, 05.07.2019 02:50




Business, 05.07.2019 02:50

History, 05.07.2019 02:50

Mathematics, 05.07.2019 02:50


Social Studies, 05.07.2019 02:50

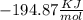
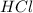 and
and 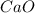 will produce
will produce 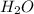 and
and 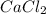 plus energy, because is an exothermic reaction. The first step is step up the reaction:
plus energy, because is an exothermic reaction. The first step is step up the reaction: