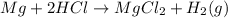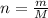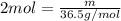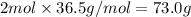Chemistry, 10.10.2019 16:50 shaloveywrighty5965
Curious carl and his lab partner were conducting a variety of experiments to produce gases: hydrogen, oxygen, and carbon dioxide. in one experiment, they added a piece of magnesium ribbon to 10 milliliters of hydrochloric acid. they observed bubbles being produced and did a variety of tests to identify the escaping gas; it proved to be hydrogen. the reaction is represented by the following equation:
mg + 2hcl → mgcl2 + h2(g)
what is the mass, in grams, of two moles of hcl?
a) 18.3 g
b) 36.5 g
c) 72.0 g
d) 73.0 g

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
Curious carl and his lab partner were conducting a variety of experiments to produce gases: hydroge...
Questions



















Social Studies, 23.05.2020 19:59