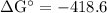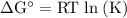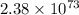Chemistry, 06.01.2020 23:31 daniella0123
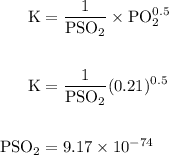
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction is -418.6 kj. what is pso2 in equilibrium with air (po2 = 0.21 atm) and solid cao?
cao(s)+so2 (g)+1⁄2o2 (g)⇔caso4 (s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

You know the right answer?
Calcium oxide is used to remove pollutant so2 from smokestack gases. the δg° of the overall reaction...
Questions



Mathematics, 28.05.2021 20:20

Mathematics, 28.05.2021 20:20



Mathematics, 28.05.2021 20:20







Chemistry, 28.05.2021 20:20


World Languages, 28.05.2021 20:20

Computers and Technology, 28.05.2021 20:20


Mathematics, 28.05.2021 20:20

Chemistry, 28.05.2021 20:20

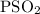 for the given reaction is
for the given reaction is 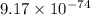 atm.
atm.