c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1

Chemistry, 02.09.2019 16:00 emily200705
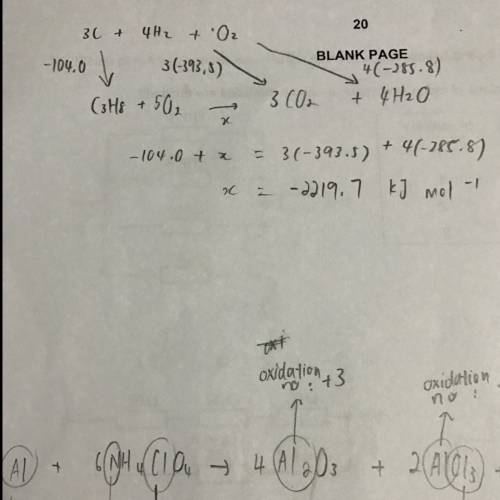
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
h2(g) + o2(g) → h2o(l) ∆h = −285.8 kj mol−1
3c(s) + 4h2(g) → c3h8(g) ∆h = −104.0 kj mol−1
4c(s) + 5h2(g) → c4h10(g) ∆h = −125.2 kj mol−1
the value in kj mol−1
for the enthalpy of combustion of propane is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

You know the right answer?
Use the information below to answer this question
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
c(s) + o2(g) → co2(g) ∆h = −393.5 kj mol−1
Questions

Mathematics, 24.04.2021 20:00


Mathematics, 24.04.2021 20:00

Mathematics, 24.04.2021 20:00

Physics, 24.04.2021 20:00

Mathematics, 24.04.2021 20:00

Social Studies, 24.04.2021 20:00


Mathematics, 24.04.2021 20:00

Mathematics, 24.04.2021 20:00


Mathematics, 24.04.2021 20:00


Computers and Technology, 24.04.2021 20:00






Physics, 24.04.2021 20:00