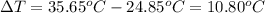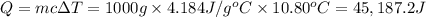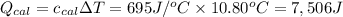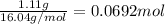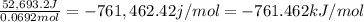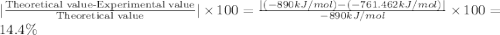To begin the experiment, 1.11g of methane ch4 is burned in a bomb
calorimeter containing 1000 grams of water. the initial temperature of water is 24.85oc. the specific heat of water is 4.184 j/g oc. the heat capacity of the calorimeter is 695 j/ oc . after the reaction the final temperature of the water is 35.65oc.
2. calculate the change in temperature, δt
3. using the formula qwater = m • c • δt ,calculate the heat absorbed by the water.
4. using the formula qcal = ccal • δt ,calculate the heat absorbed by the calorimeter.
5. the total heat absorbed by the water and the calorimeter can be calculated
by adding the heat calculated in steps 3 and 4. the amount of heat released
by the reaction is equal to the amount of heat absorbed with the negative
sign as this is an exothermic reaction. using the formula ∆h = -(qcal qwater + ) ,
calculate the total heat of combustion.
6. evaluate the information contained in this calculation and complete the
following sentence:
this calculation shows that burning grams of methane [takes
in/gives off] energy.
7. the molar mass of methane is 16.04 g/mol. calculate the number of moles
of methane burned in the experiment.
8. what is the experimental molar heat of combustion?
9. the accepted value for the heat of combustion of methane is -890 kj/mol.
explain why the experimental data might differ from the theoretical value.
10.give the formula theoretical value - experimental value % error = ×100
theoretical value , calculate
the percent error for the experiment.
with any of

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
To begin the experiment, 1.11g of methane ch4 is burned in a bomb
calorimeter containing 1000...
calorimeter containing 1000...
Questions










Mathematics, 23.07.2020 21:01




Geography, 23.07.2020 21:01


Mathematics, 23.07.2020 21:01

Computers and Technology, 23.07.2020 21:01


Computers and Technology, 23.07.2020 21:01

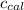 = 695 J/ °C
= 695 J/ °C