
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values for free energy and enthalpy at 25.0°c.
g = 130.5 kj/mol
h = 178.3 kj/mol
what is the entropy of the reaction? use g = h – ts.
a. -160.3 j/(mol. k)
b. -47.8 j/(mol. k)
c. 160.3 j/(mol. k)
d. 1,912 j/(mol. k)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
The decomposition of calcium carbonate, caco3(s) --> cao(s) + co2(g), has the following values f...
Questions


History, 15.07.2019 02:00



Mathematics, 15.07.2019 02:00



Mathematics, 15.07.2019 02:00






Mathematics, 15.07.2019 02:00

Mathematics, 15.07.2019 02:00



Mathematics, 15.07.2019 02:00


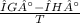
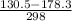 = 0.1603kJ/(mol.K) = 160.3kJ/(mol.K)
= 0.1603kJ/(mol.K) = 160.3kJ/(mol.K) 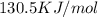
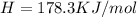
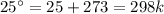
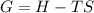
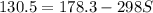

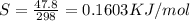
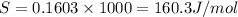 (1KJ=1000J)
(1KJ=1000J)


