
Chemistry, 27.09.2019 04:00 jorfos7683
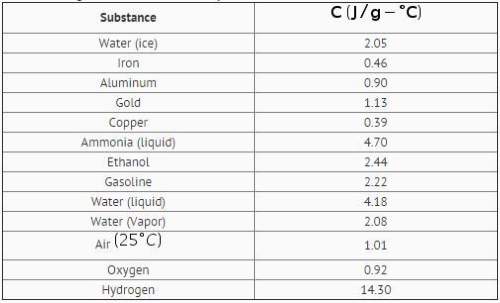
Two hundred grams of a substance requires 9.76kj of heat to raise its temperature from 25 c to 45 c. it has a specific heat of 2.44j/g. use the table to identify the substance.
a ethanol
b gasoline
c ice
d air
i think it's gasoline!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Two hundred grams of a substance requires 9.76kj of heat to raise its temperature from 25 c to 45 c....
Questions


Geography, 22.08.2019 18:00

History, 22.08.2019 18:00


English, 22.08.2019 18:00

Mathematics, 22.08.2019 18:00

History, 22.08.2019 18:00


Social Studies, 22.08.2019 18:00

English, 22.08.2019 18:00

Mathematics, 22.08.2019 18:00

English, 22.08.2019 18:00


History, 22.08.2019 18:00

English, 22.08.2019 18:00


Mathematics, 22.08.2019 18:00

Social Studies, 22.08.2019 18:00


Social Studies, 22.08.2019 18:00



