
Chemistry, 08.01.2020 20:31 claudia122752
When sodium is excited in a flame, two ultraviolet spectral lines at lambda - 372.1 nm and lambda = 376.4 nm respectively are emitted . which wavelength represented photons?
a) higher energy?
b) longer wavelengths?
c) higher frequences?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 09:20
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1
You know the right answer?
When sodium is excited in a flame, two ultraviolet spectral lines at lambda - 372.1 nm and lambda =...
Questions

History, 31.12.2019 04:31



Chemistry, 31.12.2019 04:31



Biology, 31.12.2019 04:31

Mathematics, 31.12.2019 04:31




Mathematics, 31.12.2019 04:31



Chemistry, 31.12.2019 04:31





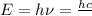 (Planck's equation)
(Planck's equation)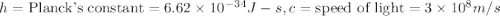
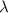 = wavelength of the photon with energy E in meters.
= wavelength of the photon with energy E in meters. = frequency of the photon with energy E in hertz.
= frequency of the photon with energy E in hertz.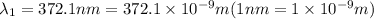
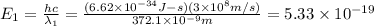 joules
joules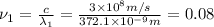 Hertz
Hertz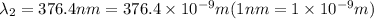
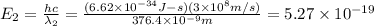 joules
joules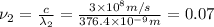 Hertz
Hertz


