
Chemistry, 25.09.2019 23:30 adrianaa34
How many moles of potassium oxide (k2o) will be formed when 1.52 moles of potassium reacts with oxygen according to the following reaction : 4 k + o2 = k2o

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
How many moles of potassium oxide (k2o) will be formed when 1.52 moles of potassium reacts with oxyg...
Questions

Social Studies, 12.01.2021 18:10


Mathematics, 12.01.2021 18:10

World Languages, 12.01.2021 18:10

Business, 12.01.2021 18:10

Physics, 12.01.2021 18:10

Chemistry, 12.01.2021 18:10

Mathematics, 12.01.2021 18:10

Mathematics, 12.01.2021 18:10

Computers and Technology, 12.01.2021 18:10

Biology, 12.01.2021 18:10

Mathematics, 12.01.2021 18:10

Social Studies, 12.01.2021 18:10

Mathematics, 12.01.2021 18:10



Mathematics, 12.01.2021 18:10



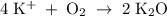
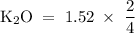 moles
moles 0.5 moles
0.5 moles

