
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to establish the following equilibrium:
i2(g) + br2(g) 2ibr(g)
keq = 280
which one of the following relates [ibr] to [i2] at equilibrium?
[i2] = [ibr]
[i2] < [ibr]
[i2] = 2 [ibr]
[i2] = 280 [ibr]

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
An equal number of moles of i2(g) and br2(g) are placed into a closed container and allowed to estab...
Questions


History, 22.01.2021 16:30




English, 22.01.2021 16:30

Mathematics, 22.01.2021 16:30

Mathematics, 22.01.2021 16:30

Mathematics, 22.01.2021 16:30

Biology, 22.01.2021 16:30

Computers and Technology, 22.01.2021 16:30

Business, 22.01.2021 16:30


Mathematics, 22.01.2021 16:30

English, 22.01.2021 16:30

Mathematics, 22.01.2021 16:30



History, 22.01.2021 16:30

Health, 22.01.2021 16:30

![[I_2]](/tpl/images/0244/0546/a6faa.png)
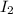 = Moles of
= Moles of 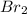


![K_{eq}=\frac{[IBr]^2}{[I_2][Br_2]}](/tpl/images/0244/0546/86934.png)
![[IBr]](/tpl/images/0244/0546/f07e4.png) and
and ![[I_2]](/tpl/images/0244/0546/a1c01.png) .
.![280=\frac{[IBr]^2}{[I_2][I_2]}](/tpl/images/0244/0546/a9dc3.png)
![280=\frac{[IBr]^2}{[I_2]^2}](/tpl/images/0244/0546/b309d.png)
![\sqrt{280}=\frac{[IBr]}{[I_2]}](/tpl/images/0244/0546/a31b5.png)
![[IBr]=16.733\times [I_2]](/tpl/images/0244/0546/79021.png)
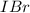 is greater than the concentration of
is greater than the concentration of 

