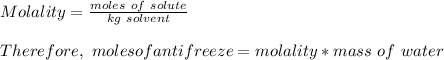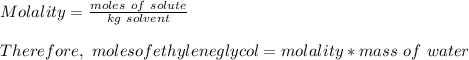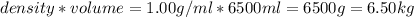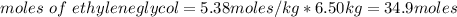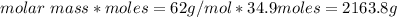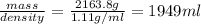Chemistry, 26.09.2019 06:00 inglehailey
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is -10.°c? (the density of ethylene glycol is 1.11 g/ml. assume the density of water at -10.°c is 1.00 g/ml.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator c...
Questions


Mathematics, 11.03.2021 17:00




Mathematics, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00


Business, 11.03.2021 17:00




Social Studies, 11.03.2021 17:00


Mathematics, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00


English, 11.03.2021 17:00

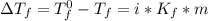
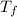 = freezing pt of solution = -10.0 C
= freezing pt of solution = -10.0 C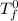 = freezing pt of pure solvent = 0 C
= freezing pt of pure solvent = 0 C![[0-(-10.0)] C= 1*(1.86 C/m) *( m)\\\\m = 5.38](/tpl/images/0263/9231/f27db.png)