
What ratio is used to carry out each conversion? a. mol ch4 to grams ch4 b. l ch4(g) to mol ch4(g)(at stp) c. molecules ch4 to mol ch4 i honestly don't kno what it's asking me i got 1 mol ch4/ 16(g) ch4 for a. 1 mol/22.4 l for b. and 6.02 x 10^23 molecules/1 mol but i don't think this is right, is it?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
What ratio is used to carry out each conversion? a. mol ch4 to grams ch4 b. l ch4(g) to mol ch4(g)(...
Questions

Physics, 19.12.2019 00:31

Geography, 19.12.2019 00:31


Biology, 19.12.2019 00:31


English, 19.12.2019 00:31

Mathematics, 19.12.2019 00:31

Mathematics, 19.12.2019 00:31

Business, 19.12.2019 00:31

Mathematics, 19.12.2019 00:31








Mathematics, 19.12.2019 00:31

Physics, 19.12.2019 00:31

Social Studies, 19.12.2019 00:31

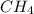 Moles to
Moles to 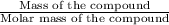
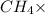 16 g/mol
16 g/mol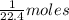
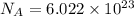 atoms or molecules
atoms or molecules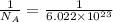 mole.
mole.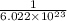 mole to get the number of moles of
mole to get the number of moles of 

