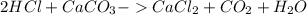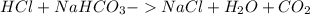The main acid in the stomach is hydrochloric acid (hcl). which chemical equations show a reaction that occurs when someone takes an antacid? check all that apply.
hcl + naoh -> nacl + h2o
2hcl + caco3 -> cacl2 + co2 + h2o
2hcl + mg(oh)2 -> mgcl2 + 2h2o
6hcl + 2al -> 2alcl3 + 3h2
hcl + nahco3 -> nacl + h2o + co2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
The main acid in the stomach is hydrochloric acid (hcl). which chemical equations show a reaction th...
Questions




Physics, 16.12.2020 19:30



Physics, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30




German, 16.12.2020 19:30




Mathematics, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30



History, 16.12.2020 19:30