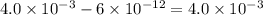The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen ions. a basic solution has a ph of 11.2. an acidic solution has a ph of 2.4. what is the approximate difference in the concentration of hydrogen ions between the two solutions? a) 1.6*10^-9 b) 4.0*10^-3 c)6.7*10^-1 d)1.6*10^-11

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
The equation for the ph of a substance is ph = –log[h+], where h+ is the concentration of hydrogen i...
Questions


Mathematics, 01.11.2020 22:30


Social Studies, 01.11.2020 22:30



Advanced Placement (AP), 01.11.2020 22:30


Biology, 01.11.2020 22:30

Mathematics, 01.11.2020 22:30




Mathematics, 01.11.2020 22:30

Computers and Technology, 01.11.2020 22:30


Social Studies, 01.11.2020 22:30


History, 01.11.2020 22:30

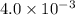
![pH=-\log [H^+]](/tpl/images/0451/5539/37e81.png)
![pH=-\log[H^+]](/tpl/images/0451/5539/cf945.png)
![11.2=-\log[H^+]](/tpl/images/0451/5539/fa6ad.png)
![[H^+]=6\times 10^{-12}M](/tpl/images/0451/5539/5538b.png)
![2.4=-\log[H^+]](/tpl/images/0451/5539/156c9.png)
![[H^+]=4\times 10^{-3}M](/tpl/images/0451/5539/9bba1.png)
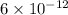 is very less as comapred to
is very less as comapred to