Chemistry, 13.12.2019 17:31 jladinosolarsee
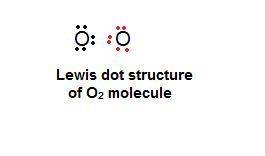
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?
one atom can lose two electrons so that the other atom can gain them and have eight valence electrons.
one atom can lose four electrons to the environment so that a total of eight valence electrons remains.
each atom can share two electrons with the other so that each atom has eight valence electrons.
each atom can lose two electrons so that there is a total of eight valence electrons between the atoms.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2


Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
What best explains how two oxygen atoms, each with six valence electrons, can bond with each other?...
Questions



Mathematics, 19.12.2020 05:50

Mathematics, 19.12.2020 05:50




Social Studies, 19.12.2020 05:50

Mathematics, 19.12.2020 05:50

Law, 19.12.2020 05:50


Arts, 19.12.2020 05:50

Mathematics, 19.12.2020 05:50

Physics, 19.12.2020 05:50



Chemistry, 19.12.2020 05:50

Mathematics, 19.12.2020 05:50

Business, 19.12.2020 05:50

Physics, 19.12.2020 05:50

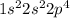
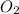 molecule is given in the image attached.
molecule is given in the image attached.