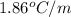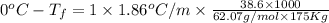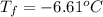Chemistry, 10.10.2019 19:30 jalenshayewilliams
Ethylene glycol, c2h6o2, is a nonvolatile substance unable to form ions in water. if 38.6 grams of ethylene glycol is dissolved in 175 grams of water, what is the freezing point of the solution? kf = 1.86°c/m; kb = 0.512°c/m
-6.61°c
1.82°c
6.61°c
-1.82°c

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Ethylene glycol, c2h6o2, is a nonvolatile substance unable to form ions in water. if 38.6 grams of e...
Questions



History, 29.09.2019 09:30


Mathematics, 29.09.2019 09:30



English, 29.09.2019 09:30


History, 29.09.2019 09:30

Mathematics, 29.09.2019 09:30

Social Studies, 29.09.2019 09:30

English, 29.09.2019 09:30


Biology, 29.09.2019 09:30


Mathematics, 29.09.2019 09:30

Mathematics, 29.09.2019 09:30


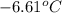
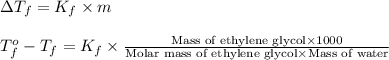
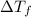 = change in freezing point
= change in freezing point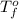 = temperature of pure water =
= temperature of pure water = 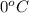
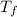 = temperature of solution = ?
= temperature of solution = ?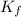 = freezing point constant =
= freezing point constant =