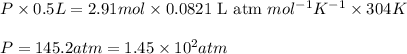Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Given 2.91 moles of a gas in a 500 milliliter-container, if the temperature is found to be 31 degree...
Questions











Health, 29.07.2019 19:00





Mathematics, 29.07.2019 19:00

Biology, 29.07.2019 19:00


Biology, 29.07.2019 19:00

Mathematics, 29.07.2019 19:00

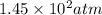
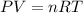
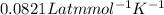
![31^oC=[273+31]K=304K](/tpl/images/0304/4872/a160b.png)