Chemistry, 16.09.2019 15:50 conyabrew82
Propane camping stoves produce heat by the combustion of gaseous propane (c3h8). balance the skeletal equation for the combustion of propane.
c3h8(g)+o2(g)→co2(g)+h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1



Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2
You know the right answer?
Propane camping stoves produce heat by the combustion of gaseous propane (c3h8). balance the skeleta...
Questions



Biology, 26.10.2020 03:50

English, 26.10.2020 03:50

World Languages, 26.10.2020 03:50



Mathematics, 26.10.2020 03:50




English, 26.10.2020 04:00

History, 26.10.2020 04:00

English, 26.10.2020 04:00

Mathematics, 26.10.2020 04:00

Mathematics, 26.10.2020 04:00


Mathematics, 26.10.2020 04:00

World Languages, 26.10.2020 04:00

Mathematics, 26.10.2020 04:00

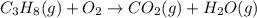
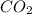 by 3 and
by 3 and 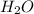 by 4 on product side by 2. Hence, the complete balanced chemical equation will be as follows.
by 4 on product side by 2. Hence, the complete balanced chemical equation will be as follows.