Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
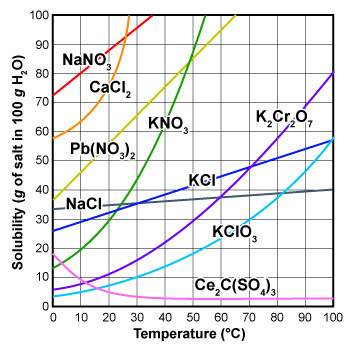
At which temperature would a 40-gram kcl per 100 grams of water solution, be considered supersaturat...
Questions


History, 08.01.2020 23:31


Mathematics, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31





Computers and Technology, 08.01.2020 23:31


Mathematics, 08.01.2020 23:31

History, 08.01.2020 23:31

Social Studies, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31


Mathematics, 08.01.2020 23:31

Mathematics, 08.01.2020 23:31