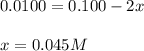10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solution of a substance l. the following equilibrium is established:
m2+(aq) + 2l(aq) picture ml22+(aq)
at equilibrium the concentration of l is found to be 0.0100 mol l–1. what is the equilibrium concentration of ml22+, in mol l–1?
someone me

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 13:40
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
You know the right answer?
10.0 ml of a 0.100 mol l–1 solution of a metal ion m2+ is mixed with 10.0 ml of a 0.100 mol l–1 solu...
Questions


Mathematics, 22.01.2020 03:31



Advanced Placement (AP), 22.01.2020 03:31

Mathematics, 22.01.2020 03:31




History, 22.01.2020 03:31

Computers and Technology, 22.01.2020 03:31


Mathematics, 22.01.2020 03:31

World Languages, 22.01.2020 03:31






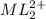 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.![[M^{2+}]_{initial}=0.100M](/tpl/images/0204/7051/8debc.png)
![[L]_{initial}=0.100M](/tpl/images/0204/7051/9e7cd.png)
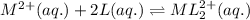
![[L]_{eqllm}=0.0100M](/tpl/images/0204/7051/18682.png)