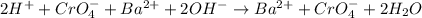What is the total ionic equation for the following reaction?
h2cro4 + ba(oh)2
h2cro4–...


Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

You know the right answer?
Questions



Mathematics, 18.03.2021 21:00




Mathematics, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00


Chemistry, 18.03.2021 21:00



Mathematics, 18.03.2021 21:00


English, 18.03.2021 21:00

Mathematics, 18.03.2021 21:00




Chemistry, 18.03.2021 21:00

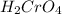 is a strong acid and
is a strong acid and 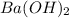 is a strong bases, therefore, both of them will dissociate into ions.
is a strong bases, therefore, both of them will dissociate into ions.