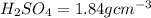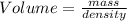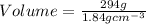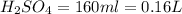2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form liquid sulfuric acid (h2so4) in the unbalanced equation below: so2 + o2 + h2o h2so4 in the laboratory, a chemist carries out this reaction at stp with 67.2 l of sulfur dioxide (so2). how many liters of h2so4 did the chemist produce? 1 mole of any gas = 22.4 l of that same gas at stp • part a: write a balanced equation for the reaction. • part b: calculate the number of liters of h2so4 produced.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1


Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
2. sulfur dioxide gas (so2) reacts with excess oxygen gas (o2) and excess liquid water (h2o) to form...
Questions

Mathematics, 14.01.2021 20:40

Biology, 14.01.2021 20:40






History, 14.01.2021 20:40




History, 14.01.2021 20:40

Chemistry, 14.01.2021 20:40

English, 14.01.2021 20:40





History, 14.01.2021 20:40

Mathematics, 14.01.2021 20:40

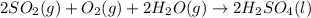
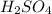 =0.16L
=0.16L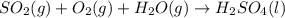
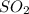 occupies 22.4 L at STP
occupies 22.4 L at STP 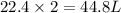 and produce 2 moles of
and produce 2 moles of 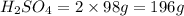
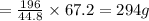 of
of