
Chemistry, 06.01.2020 20:31 cchavcchav9606
If i have 7.7 moles of gas at a pressure of 0.0990 atm and at a temperature of 256 k, what is the volume of the container that the gas is in?
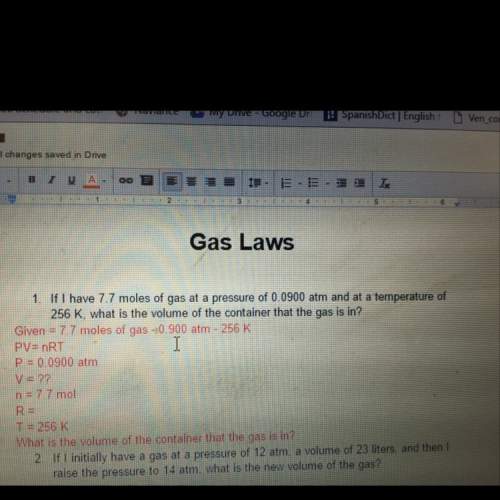

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
If i have 7.7 moles of gas at a pressure of 0.0990 atm and at a temperature of 256 k, what is the vo...
Questions



Physics, 11.11.2019 18:31





Computers and Technology, 11.11.2019 18:31


Computers and Technology, 11.11.2019 18:31












