
Chemistry, 05.01.2020 11:31 KingMack1136
If i have 7.7 moles of gas at a pressure of 0.0990 atm and at a temperature of 256 k, what is the volume of the container that the gas is in?
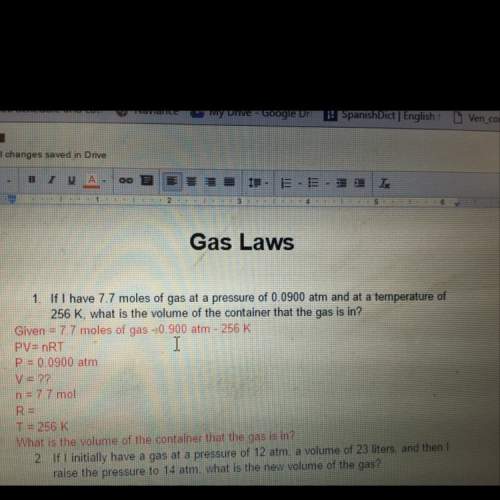

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 13:00
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2
You know the right answer?
If i have 7.7 moles of gas at a pressure of 0.0990 atm and at a temperature of 256 k, what is the vo...
Questions


Mathematics, 25.10.2019 11:43

Mathematics, 25.10.2019 11:43


History, 25.10.2019 11:43

History, 25.10.2019 11:43

Spanish, 25.10.2019 11:43

Mathematics, 25.10.2019 11:43

Mathematics, 25.10.2019 11:43

History, 25.10.2019 11:43



History, 25.10.2019 11:43


Social Studies, 25.10.2019 11:43




English, 25.10.2019 11:43

Mathematics, 25.10.2019 11:43



