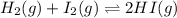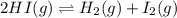Chemistry, 19.11.2019 17:31 mattydoug4818
Two experiments were performed involving the following equilibrium. the temperature was the same in both experiments. h2(g) + i2(g) 2hi(g) in experiment a, 1.0 m i2 and 1.0 m h2 were initially added to a flask and equilibrium was established. in experiment b, 2.0 m hi was initially added to a second flask and equilibrium was established. which of the following statements is always true about the equilibrium concentrations?
a.[h2] equals [hi] in experiment a.
b.[hi] equals 2[h2] in experiment a.
c.[hi] in experiment a equals [hi] in experiment b.
d.[hi] in experiment a equals 1/2[i2] in experiment b.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
Two experiments were performed involving the following equilibrium. the temperature was the same in...
Questions



Social Studies, 11.12.2020 09:30

Mathematics, 11.12.2020 09:30




Mathematics, 11.12.2020 09:40


Mathematics, 11.12.2020 09:40

Mathematics, 11.12.2020 09:40


Mathematics, 11.12.2020 09:40


Advanced Placement (AP), 11.12.2020 09:40



Biology, 11.12.2020 09:40


Mathematics, 11.12.2020 09:40