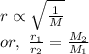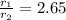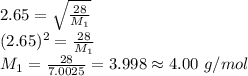Chemistry, 16.10.2019 17:40 cecilysimpson7521
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressure of each gas is known to be 200 torr at 25°c. the gases are allowed to effuse through a pinhole, and it is found that gas a escapes 2.65 times the rate of nitrogen gas. what is the molar mass of gas a?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1
You know the right answer?
Asample of nitrogen gas is contaminated with a gas (gas a) of unknown molar mass. the partial pressu...
Questions


Chemistry, 11.11.2020 01:30

Mathematics, 11.11.2020 01:30

Mathematics, 11.11.2020 01:30

Mathematics, 11.11.2020 01:30




History, 11.11.2020 01:30

History, 11.11.2020 01:30


SAT, 11.11.2020 01:40

Mathematics, 11.11.2020 01:40

Mathematics, 11.11.2020 01:40

Advanced Placement (AP), 11.11.2020 01:40

History, 11.11.2020 01:40


Biology, 11.11.2020 01:40

Mathematics, 11.11.2020 01:40