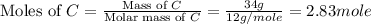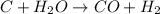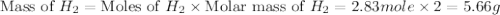Chemistry, 12.01.2020 19:31 ausemkattom3034
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o? the reaction is:
c + h2o → co + h2
the atomic mass of c is 12.01 g/mole. the atomic mass of h2 is 2.016 g/mole. finish the problem by choosing the correct format for dimensional analysis.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

You know the right answer?
How many grams of h2 would be formed if 34 grams of carbon reacted with an unlimited amount of h2o?...
Questions

Mathematics, 07.05.2021 03:30

Mathematics, 07.05.2021 03:30


English, 07.05.2021 03:30

Mathematics, 07.05.2021 03:30

SAT, 07.05.2021 03:30



Mathematics, 07.05.2021 03:30

English, 07.05.2021 03:30


Mathematics, 07.05.2021 03:30

Mathematics, 07.05.2021 03:30


Physics, 07.05.2021 03:30




Mathematics, 07.05.2021 03:30

Mathematics, 07.05.2021 03:30

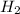 will be, 5.66 grams
will be, 5.66 grams