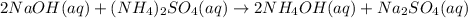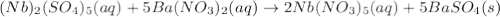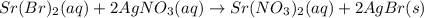Chemistry, 06.10.2019 13:00 genyjoannerubiera
Predict whether each of the following reactions will occur in aqueous solutions. explain your reasoning in each case. note: barium sulfate and silver bromide precipitate in aqueous solutions.
(a) sodium hydroxide + ammonium sulfate → ?
(b) niobium(v) sulfate + barium nitrate → ?
(c) strontium bromide + silver nitrate → ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1


Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Predict whether each of the following reactions will occur in aqueous solutions. explain your reason...
Questions



Mathematics, 03.09.2020 14:01


Chemistry, 03.09.2020 14:01

World Languages, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01



Mathematics, 03.09.2020 14:01

English, 03.09.2020 14:01

Law, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01


Physics, 03.09.2020 14:01

Biology, 03.09.2020 14:01


Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01

Mathematics, 03.09.2020 14:01