
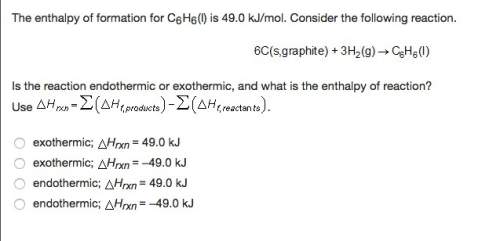
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
is the reaction endothermic or exothermic, and what is the enthalpy of reaction?
exothermic; mc014-3.jpghrxn = 49.0 kj
exothermic; mc014-4.jpghrxn = –49.0 kj
endothermic; mc014-5.jpghrxn = 49.0 kj
endothermic; mc014-6.jpghrxn = –49.0 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2
You know the right answer?
The enthalpy of formation for c6 h6 (i) is 49.0 kj/mol. consider the following reaction.
...
...
Questions




Mathematics, 03.02.2021 23:20



Social Studies, 03.02.2021 23:20



Mathematics, 03.02.2021 23:20

Engineering, 03.02.2021 23:20



Mathematics, 03.02.2021 23:20

Chemistry, 03.02.2021 23:20

History, 03.02.2021 23:20

Mathematics, 03.02.2021 23:20

Mathematics, 03.02.2021 23:20


Mathematics, 03.02.2021 23:20

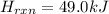
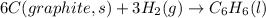
![\Delta H=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0345/6718/76c37.png)
![\Delta H=[(n_{C_6H_6}\times \Delta H_{C_6H_6})]-[(n_{H_2}\times \Delta H_{H_2})+(n_{C}\times \Delta H_{C})]](/tpl/images/0345/6718/5d909.png)
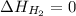 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
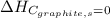 (as heat of formation of substances in their standard state is zero
(as heat of formation of substances in their standard state is zero
![\Delta H=[(1\times 49.0)]-[(3\times 0)+(6\times 0]](/tpl/images/0345/6718/8bb49.png)
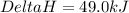
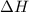 for the reaction comes out to be negative.
for the reaction comes out to be negative.



