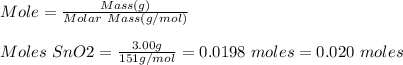Chemistry, 03.02.2020 21:02 alyssamaize
Base your answer to the question on the information below and on your knowledge of chemistry. at 1023 k and 1 atm, a 3.00-gram sample of sno2(s) (gram formula mass = 151 g/mol) reacts with hydrogen gas to produce tin and water, as shown in the balanced equation below. sno2(s) + 2h2(g) → sn(l) + 2h2o(g) show a numerical setup for calculating the number of moles of sno2(s) in the 3.00-gram sample.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Base your answer to the question on the information below and on your knowledge of chemistry. at 102...
Questions

Chemistry, 18.09.2019 14:20

Mathematics, 18.09.2019 14:20

Health, 18.09.2019 14:20




English, 18.09.2019 14:20


Mathematics, 18.09.2019 14:20


Mathematics, 18.09.2019 14:20

Mathematics, 18.09.2019 14:20

Biology, 18.09.2019 14:20



Mathematics, 18.09.2019 14:20


Biology, 18.09.2019 14:20

Mathematics, 18.09.2019 14:20