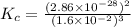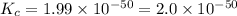Chemistry, 24.09.2019 13:30 genyjoannerubiera
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of o3 is 2.86 x 10-28 m. what is the equilibrium constant of the reaction at this temperature?
a. 2.0*10∧-50
b. 2.0*10∧50
c. 1.8*10∧-26
d. 1.8*10∧26


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of...
Questions












Business, 21.07.2019 04:00




Mathematics, 21.07.2019 04:00



Advanced Placement (AP), 21.07.2019 04:00

Physics, 21.07.2019 04:00

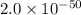
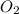 =
= 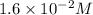
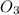 =
= 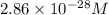
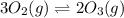
![K_c=\frac{[O_3]^2}{[O_2]^3}](/tpl/images/0258/2319/13f9f.png)