
Chemistry, 15.10.2019 01:30 Justinoreilly71
The reason iodine, i2, exists as a solid at room temperature, while bromine, br2, exists as a liquid at the same temperature, is because:
iodine exhibits hydrogen bonding, while bromine exhibits only london dispersion forces.
iodine molecules create stronger london dispersion forces than do bromine molecules.
bromine exists only as a gas at all temperatures.
bromine forms fewer dipole-dipole interactions than does iodine.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
The reason iodine, i2, exists as a solid at room temperature, while bromine, br2, exists as a liquid...
Questions


Mathematics, 27.08.2020 22:01


History, 27.08.2020 22:01

Physics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01


Mathematics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01


English, 27.08.2020 22:01

Arts, 27.08.2020 22:01


Mathematics, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01

History, 27.08.2020 22:01



Social Studies, 27.08.2020 22:01

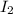 , exists as a solid at room temperature, while bromine,
, exists as a solid at room temperature, while bromine, 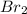 , exists as a liquid at the same temperature, is because: Iodine molecules create stronger London dispersion forces than do bromine molecules.
, exists as a liquid at the same temperature, is because: Iodine molecules create stronger London dispersion forces than do bromine molecules.

