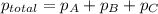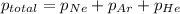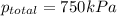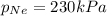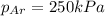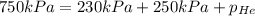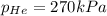Chemistry, 11.12.2019 09:31 rhyanebean6443
The total pressure inside a vessel containing a mixture of neon, argon, and helium gases is 750 kpa. the partial pressure of neon is 230 kpa, and the partial pressure of argon is 250 kpa. what is the partial pressure of helium?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

You know the right answer?
The total pressure inside a vessel containing a mixture of neon, argon, and helium gases is 750 kpa....
Questions

Spanish, 07.07.2019 21:30


History, 07.07.2019 21:30

English, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

Business, 07.07.2019 21:30

History, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

Biology, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

History, 07.07.2019 21:30


Mathematics, 07.07.2019 21:30


Chemistry, 07.07.2019 21:30

History, 07.07.2019 21:30