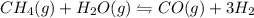Chemistry, 15.12.2019 01:31 montgomerykarloxc24x
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)
what will happen to the equilibrium of this reaction if the pressure is increased?
a. the equilibrium will shift to the left to favor the reverse reaction.
b. the equilibrium will shift to the right to favor the forward reaction.
c. the equilibrium will not be affected by changing the pressure.
d. the equilibrium will not be reestablished after this kind of stress.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Consider the chemical equation in equilibrium.
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
ch4(g) + h2o(g) < > co(g) + 3h2(g)...
Questions

Chemistry, 03.05.2021 23:40

World Languages, 03.05.2021 23:40

Computers and Technology, 03.05.2021 23:40

Mathematics, 03.05.2021 23:40



English, 03.05.2021 23:40

History, 03.05.2021 23:40



Computers and Technology, 03.05.2021 23:40


Mathematics, 03.05.2021 23:40




Chemistry, 03.05.2021 23:40

Biology, 03.05.2021 23:40