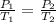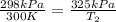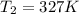Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

You know the right answer?
The pressure in a car tire is 298 kilopascals at 300 kelvin. after a long drive, the pressure become...
Questions


World Languages, 18.10.2021 01:30


Mathematics, 18.10.2021 01:40


English, 18.10.2021 01:40



Mathematics, 18.10.2021 01:40





Mathematics, 18.10.2021 01:40

Biology, 18.10.2021 01:40

Mathematics, 18.10.2021 01:40



Mathematics, 18.10.2021 01:40

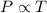 (At constant volume and number of moles)
(At constant volume and number of moles)