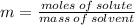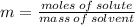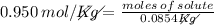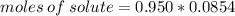Chemistry, 10.10.2019 12:30 raymondanthony6567
The molality of a solution that is made by dissolving a certain mass of ethanol in 85.4 g of water is 0.950 m. how many moles of ethanol were dissolved?
0.0811 mol
0.0899 mol
11.1 mol
81.1 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The molality of a solution that is made by dissolving a certain mass of ethanol in 85.4 g of water i...
Questions


Mathematics, 22.10.2020 22:01



Mathematics, 22.10.2020 22:01

Chemistry, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Spanish, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


Engineering, 22.10.2020 22:01





Computers and Technology, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Advanced Placement (AP), 22.10.2020 22:01


Arts, 22.10.2020 22:01